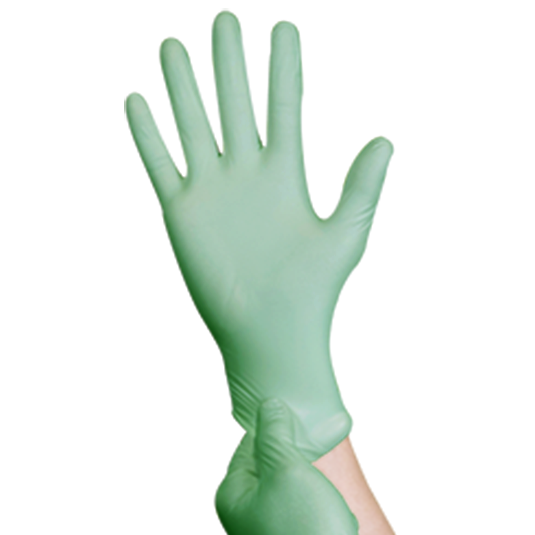
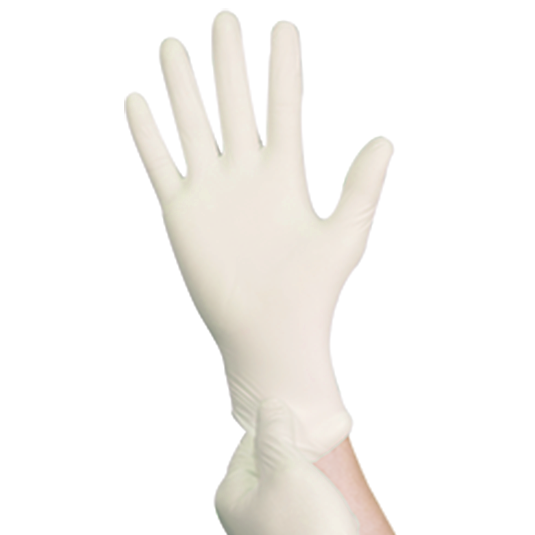
BIOGREEN™ Biodegradable Nitrile Powder Free Glove
One of Top Glove's ESG initiatives in creating a sustainable world. It provides an "end of life solution" to landfills by reducing the accumulation of waste. Specially formulated to biodegrade in biologically active landfills through a microbial process. Proudly giving businesses more options with an additional feature of Accelerator Free to minimise the risk of Type IV Delayed Hypersensitivity reaction. This is the most popular and reliable glove for medical, healthcare and dental industries. This glove is a reliable protection to avoid cross contamination between food and caterers. A dependable protection for tougher jobs including laboratory work, cleaning, maintenance and general applications. Made of premium compounded nitrile eliminating latex allergy concern. It comes with excellent fit, comfort, sensitivity and protection. Its textured surface helps to provide secure grip and added handling precision.
Colour